Researchers Achieve First-Ever 3D Real-Time Imaging of Human Embryo Implantation
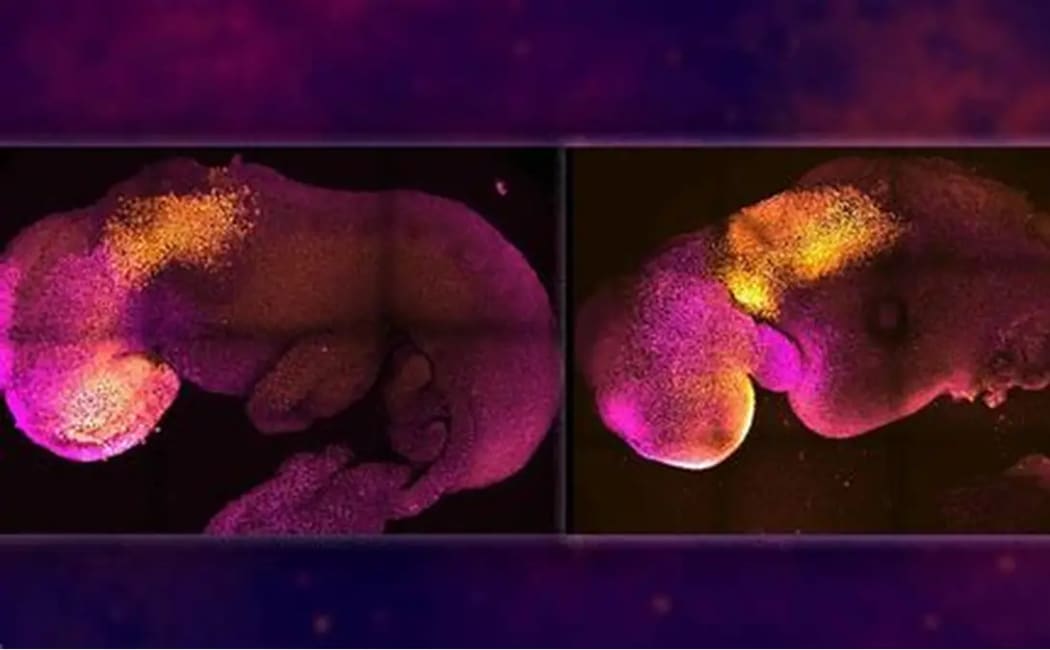
Researchers Capture First 3D Real-Time Images of Human Embryo Implantation
In a groundbreaking scientific advancement, researchers from the Institute for Bioengineering of Catalonia (IBEC), in collaboration with Dexeus University Hospital, have successfully recorded the first-ever 3D real-time images of a human embryo implanting into the uterus. This achievement marks a historic milestone in reproductive health and fertility research, providing valuable insights into one of the most mysterious phases of early human development.
Why Implantation Matters in Fertility
Implantation is a critical stage in pregnancy where the embryo embeds itself into the uterine wall. Failure at this stage accounts for nearly 60% of spontaneous miscarriages and remains one of the leading causes of infertility. Until now, scientists could only rely on still images and partial observations, leaving many unanswered questions about the precise mechanics of the process.
The Discovery: Watching the Embryo in Action
For the first time, researchers observed how human embryos exert force as they burrow into uterine tissue. According to Dr. Samuel Ojosnegros, principal investigator of IBEC’s Bioengineering for Reproductive Health group, “Embryos need to actively invade uterine tissue to fully integrate. This is a surprisingly invasive process, explaining why some women may feel abdominal discomfort or light bleeding during early pregnancy.”
The embryo does not rely solely on biochemical signals. It also releases enzymes to break down surrounding tissues while applying mechanical force to push through collagen-rich uterine layers. Collagen, a rigid protein found in tendons and cartilage, creates resistance that the embryo must overcome. Once implanted, the embryo begins forming specialized tissues that connect with the mother’s blood supply to ensure nourishment.
The Role of Mechanical Forces
The research revealed that embryos are far from passive — they actively remodel their environment. By pulling and reorganizing the uterine matrix, embryos create a pathway for successful implantation. Amélie Godeau, co-first author of the study, emphasized, “We observed that embryos sense and respond to external force cues. Natural uterine contractions may influence how efficiently implantation occurs.”
This discovery highlights how both biochemical and mechanical factors play a role in early pregnancy. Understanding these forces could help improve fertility treatments and increase the success rates of assisted reproductive technologies (ART).
Developing a Laboratory Platform for Study
To make this breakthrough possible, the IBEC team designed a unique laboratory platform where embryos can implant outside the uterus under controlled conditions. This setup used an artificial gel matrix made of collagen and essential proteins to replicate uterine tissue. The platform enabled real-time fluorescence imaging, allowing scientists to track both biochemical signals and mechanical forces with unprecedented detail.
By comparing human embryos with mouse embryos, researchers noticed striking differences. In mice, the uterus folds around the embryo, creating a protective crypt. In humans, however, the embryo aggressively pushes inward, penetrating deep into uterine tissue and expanding outward as development progresses. These distinctions highlight the importance of studying human embryos directly, as animal models may not always replicate human biology.
Implications for Fertility and Medicine
The ability to capture real-time 3D implantation has far-reaching implications. For couples struggling with infertility, this research could improve embryo selection methods, enhance in vitro fertilization (IVF) protocols, and potentially reduce the time it takes to conceive. By identifying the mechanical “footprint” of successful implantation, doctors may better predict which embryos are most viable.
Beyond fertility, the findings could also impact terrestrial medicine. Understanding how cells exert forces to invade tissue may offer insights into cancer research, wound healing, and regenerative medicine, where similar processes of tissue remodeling occur.
Global Collaboration Behind the Breakthrough
This pioneering study was made possible through collaboration with several institutions, including the Barcelona Stem Cell Bank (IDIBELL), the University of Barcelona (UB), Tel Aviv University, the Biomedical Research Networking Centre (CIBER), and the Institute for Research in Biomedicine (IRB Barcelona). Dexeus University Hospital donated the embryos used in the study, ensuring ethical standards were met throughout the research.
Conclusion: A New Window into Human Development
The successful imaging of human embryo implantation in 3D and real time represents a landmark achievement in reproductive science. For the first time, scientists can observe the hidden mechanics of life’s earliest stage, bringing hope to millions facing infertility and expanding our knowledge of human biology.
As Dr. Anna Seriola, co-first author of the study, summarized: “Our platform has allowed us to quantify the dynamics of implantation and identify the forces that make it possible. This knowledge paves the way for breakthroughs in fertility treatments and beyond.”
